Abstract
Introduction Due to its heterogenous biology, chronic lymphocytic leukemia (CLL) is a well-suited disease for identifying new relationships between genomic and functional biomarkers for drug response. Here, we combined a multi-omics approach and ex vivo drug-treatment data with functional characterization of apoptotic sensitivity to correlate genetic features and drug sensitivity with specific anti-apoptotic signaling patterns measured by BH3 profiling in primary CLL samples.
Methods BH3 profiling is a functional assay to measure the proximity of cells to undergo apoptosis via overall priming as well as to identify specific anti-apoptotic proteins the cells are dependent on for survival. BH3 peptides and mimetic such as BIM and PUMA were used to identify overall priming or proximity to apoptosis, BAD and ABT199 were used to identify BCL-2 dependence, and FS1, MS1 and HRK were used to identify BFL-1, MCL-1 and BCL-xL dependences respectively (Ryan et al, Biol Chem, 2016).
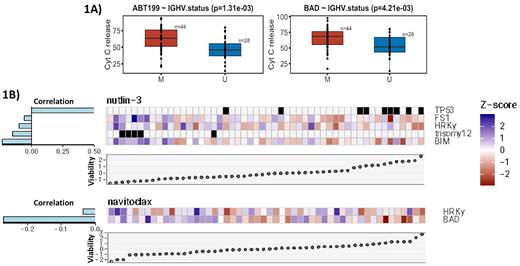
Results We analyzed primary CLL samples from 73 patients and using BH3 profiling identified the specific associations between anti-apoptotic protein dependence and genetic features (such as IGHV status, trisomy-12, del(17p) and TP53 mutation. Our univariate models demonstrated that BCL-2 dependence, as measured by ABT199 drug (p=0.001) and BAD (p=0.004) BH3 peptide, are highly correlated with mutated IGHV status, a favorable prognostic marker in CLL (Figure 1A). Using ex vivo treatment with multiple classes of small molecule inhibitors, we further identified several specific drug response or sensitivity patterns correlating with anti-apoptotic protein dependence as well as CLL prognostic factors (Figure 1B). In particular, our multivariate models (LASSO regularized) demonstrated that viability following ex vivo treatment with the MDM-2 inhibitor Nutlin-3 was positively correlated with TP53 mutation/deletion, but inversely correlated with BFL-1 (FS1 peptide) and BCL-xL (HRK peptide) dependence as well as trisomy-12 (Figure 1B), though the inverse correlation between Nutlin-3 and BFL-1 or BCL-xL dependence is relatively weak. CLL cell viability following ex vivo treatment with the dual BCL-2/BCL-xL inhibitor navitoclax was negatively correlated with BCL-2 (BAD peptide) and BCL-xL (HRK peptide) dependence, though primarily BCL-2 dependence, suggesting that sensitivity to navitoclax is highly dependent on BCL-2 (Figure 1B). At the transcriptomic level, CLL cell dependence on BCL-2 positively correlated with NOXA expression, NFκB signaling, and reduced intracellular oxidative level, various pathways that could potentially maintain BCL-2 dependence. Finally, we utilized BH3 profiling to retrospectively predict in vivo drug response, with high BCL-2 dependence (p=0.016) correlating with greater absolute lymphocyte count decrease.
Conclusion Functional characterization of apoptotic signaling in CLL demonstrated that patients with mutated IGHV are more dependent on BCL-2 for survival, and that BH3 profiling could retrospectively predict in vivo sensitivity to BCL-2 inhibition. This functional precision medicine approach may help to stratify patients with CLL beyond genetic markers and should be further explored for correlation with clinical outcomes in prospective clinical trials.
Disclosures
Valentin:Genmab: Current Employment. Lehmberg:Casma Therapeutics: Current Employment. Herbaux:Kite: Honoraria; Gilead: Honoraria; Janssen: Honoraria; Roche: Honoraria; MSD: Research Funding; Abbvie: Honoraria, Research Funding; Takeda: Honoraria, Research Funding. Niemann:Abbvie: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Janssen: Consultancy; Octapharma: Consultancy, Research Funding; CSL Behring: Consultancy; Takeda: Consultancy; Beigene: Consultancy; Genmab: Consultancy. Zenz:Janpix: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Roche: Consultancy, Honoraria. Davids:Verastem: Consultancy, Research Funding; Novartis: Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ascentage Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Eli Lilly and Company: Consultancy, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy; Research to Practice: Honoraria; Ono Pharmaceuticals: Consultancy; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses, Research Funding; Takeda: Consultancy; TG Therapeutics: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal